Abstract
Background: As molecular testing becomes more widely available, the prognostic and therapeutic implications of specific genetic mutations in acute myeloid leukemia (AML) continue to evolve. Mutations in the Neurofibromin 1 (NF1) gene are not well studied in AML. A limited number of studies have evaluated the presence and potential role of NF1 in AML (Eisfeld et al., 2018; Telfah et al., 2019). NF1 mutations were found in about 5% of AML patients and may carry a worse prognosis. In this IRB-approved retrospective case-control study, we analyzed specific clinical outcomes for AML patients with any NF1 mutation compared to patients with a wild-type NF1 gene, including complete remission (CR) rate and overall survival (OS).
Methods: We reviewed electronic medical charts of newly diagnosed AML patients who had next-generation-sequencing (NGS) from bone marrow aspirates at diagnosis at our institution between 2017-2021. We identified patients with any pathogenic or likely pathogenic NF1 mutation. Control patients with a wild-type NF1 gene were matched to the NF1 positive group based on age, sex, race, and AML risk per 2017 ELN criteria. The primary outcome was overall survival; secondary outcomes included the rate of achieving a complete remission (CR), complete remission with incomplete hematologic recovery (CRi) at bone marrow evaluation after chemotherapy induction, allogeneic hematopoietic stem cell transplant rate for those who achieved CR, and finally overall survival (OS). CR, CRi, and transplant rates were compared using a Fisher's exact test and by calculating odds ratios (OR). OS was estimated by the Kaplan-Meier method and compared by using a log-rank test. Significance was defined by a P value <0.05.
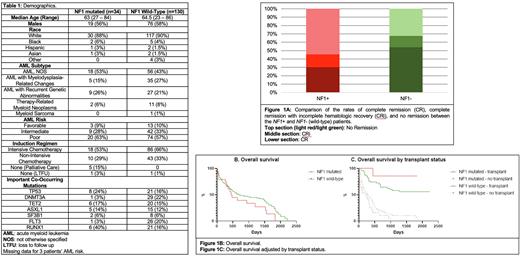
Results: We identified 34 AML patients with an NF1 mutation on NGS from initial diagnostic bone marrow aspiration between 2017-2021. We compared the outcomes with a matched control group (n=130) as described in the methods. The median age was 63 (27-84) and 64.5 (23-86) in the NF1 mutated and wild-type groups, respectively. There were no differences in sex or race between the two groups (Table 1). There were 20 patients (63%) with high-risk disease in the NF1 mutated group, similar to 74 patients (57%) in the control group. The co-occurring mutations varied between the two groups; RUNX1 mutations were more common in the NF1 mutated group, while DNMT3A and FLT3 were less common (Table 1).
NF1 mutated patients were less likely to achieve CR/CRi at 44% compared to 65% of NF1 wild-type patients (OR 0.40; P 0.03; figure 1A). Rates of subsequent allogeneic transplant for patients who achieved CR/CRi were similar in NF1 mutated and wild-type patients (66% and 60%, respectively). The median OS for NF1 mutated patients was 474 days compared to 688 days for wild-type NF1 patients (hazard ratio (HR) for death, 1.45; 95% CI, 0.88 to 2.49; P 0.15; figure 1B). There was no significant difference in median OS between patients who received allogeneic transplants, but NF1 mutated patients who did not receive a transplant had a significantly worse median OS compared to the NF1 wild type patients (HR 1.63, P 0.03; figure 1C).
Discussion: Somatic NF1 mutations are not well studied in AML. Prior limited studies are suggestive of worse outcomes, and we have observed similar results in this study. Patients with NF1 mutations were significantly less likely to achieve CR/CRi compared to patients with a wild-type NF1 gene, either with intensive or non-intensive therapies. OS was worse for NF1 mutated patients but did not reach statistical significance except for patients that did not receive an allogeneic transplant, who had significantly worse median OS. While the patients' characteristics, including the risk of AML, were similar between the NF1 mutated and wild-type groups, we observed differences in co-occurring mutations. The common DNMT3a and FLT3 mutations were significantly less common in NF1 mutated patients, while there were more RUNX1 mutations in this group. This distinctive co-occurring mutation pattern may suggest a role for NF-1 in leukemogenesis with a specific leukemia development pathway and provides an opportunity for personalized targeting.
This retrospective study has a relatively small sample size. Nonetheless, it provides further evidence for the potentially adverse prognosis of NF1 mutations in AML, which can guide conversations between treating physicians and patients about individualized prognostic risks.
Disclosures
Godwin:Sinochips Diagnostics: Current equity holder in private company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Biovica: Honoraria, Membership on an entity's Board of Directors or advisory committees; Clara Biotech: Membership on an entity's Board of Directors or advisory committees, Other: future stock options; Predicine, Inc.: Research Funding; VITRAC Therapeutics: Research Funding. Lin:AbbVie, Aptevo, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, Genentech-Roche, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Mateon Therapeutics, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seattle Genetics, Tolero, Trovagene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal